FOOD ALLERGEN TESTING
We use AgraStrip® allergen test kits from Romer Labs® for most of our customer’s allergen testing needs.
They are ready-to-use lateral flow devices designed for both laboratory and on-site testing.
Use the menu below to determine if there is a test kit available for the allergen for which you need to test.
Menu of Allergens We Test For:
The AgraStrip® Allergen lateral flow tests are for the detection of trace amounts of allergens in a low ppm range in the following samples:
- Raw materials
- Finished food samples
- Rinse water (as part of cleaning validation)
- Environmental swab samples (as part of cleaning validation)
If the sample contains more than 1% (10,000 ppm) allergen, the test will come up negative.
Results are either positive or negative, with the threshold being defined by the detection limit of each allergen type (click on each allergen to obtain the detection limit). The assay is not designed to quantify the amount of an allergen, the assay is designed to validated the absence of a given allergen in either a product sample or environmental swab.
• Allergen Testing by MBL or by Customer
The AgriStrip® test kits can be easily implemented into routine analysis as part of an allergen management plan within a HACCP-concept. The simple test format makes it easy for a smooth workflow to be maintained on-site as well as in the lab.
If you need product tested, we recommend you submit the product to us. We do not recommend in house testing of products.
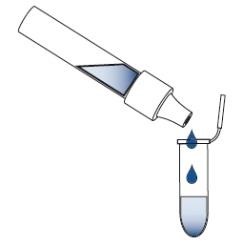
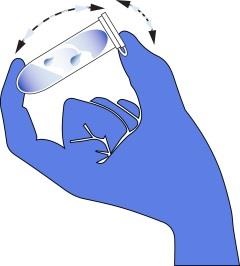
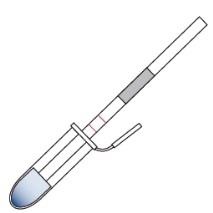
If you need to validate the absence of allergen post sanitation via rinse water or swab testing, you can do so in house. The following tabs (① through ⑤) tells you how to conduct testing on swabs. This information is from the AgraStrip® package inserts © by Romer Labs®.
Contact Romer Labs® to set up an account, order your test kits, obtain package inserts, or if you have any questions.
If you conduct your own testing, be sure to record your results.
① Important Advice for the Proper Execution of the Test:
- Each AgriStrip® Allergen kit has an extraction buffer which is specific to that kit. You cannot use the extraction buffer for 1 type of kit for any other type of allergen kit.
- AgriStrip® Allergen kits including all the reagents should be tempered to room temperature before use; the kits should not be used at refrigeration temperatures. Otherwise, when not in use, store the kits at the temperature stated by the manufacturer.
- Residual sanitizers do not affect the assays. When testing surfaces for the absence of allergen, it is okay if they are still wet from the sanitation process providing it does not alter the pH outside of the 6-8 range.
- Conduct the test in a clean area that is free of the allergen.
- It is important to read the results immediately after the 5 minute incubation step since the AgriStrip® test system has been validated extensively and shows reliable results after that exact time. Longer incubation times can lead to the development of false positive results.
- The AgriStrip® Allergen lateral flow tests are for the detection of trace amounts of allergens in a low ppm range. If the sample contains more than 1% (10,000 ppm) allergen, the test will come up negative.
- Performing the assay in a pH range of 6-8 will lead to reliable results. Highly acidic samples can lead to false positive results whereas in an alkaline medium there is the tendency to generate false negative results.
② Materials Supplied with the Kit
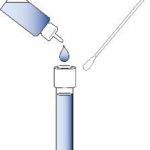
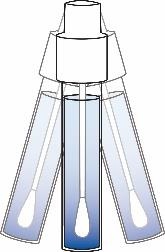
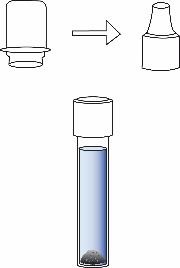
③ Sample Preparation – Swab Testing
Special Note for Gluten G12 Testing
The procedure to detect Gluten G12 using AgraStrip® is somewhat different than described above. Analyze according to the package insert.